By Bryan Quoc Le | 150 Food Science Questions Answered
You are what you eat, or so the old adage goes.
But unlike other primates and animals, humans are the only animals who cook their food, which may have more to do with how we became humans in the first place.
Richard Wrangham, Ruth Moore Professor of Biological Anthropology at Harvard University and MacArthur Fellow, has proposed the hypothesis that cooking has actually played a bigger role in our ancestral development than first believed. In his book, Catching Fire: How Cooking Made Us Human, he argues that the invention of fire, and subsequent use of fire to cook food, helped steer the evolution of the human brain from two million years ago to where it is today [1]. He shares evidence that cooking provided more nutrients to early fire-using hominids, or members of the great ape family, than the raw foods eaten by other hominids. By breaking down indigestible carbohydrates, tenderizing meat proteins, and deactivating toxins, more diverse foods became available to early humans while also requiring less energy to digest, after utilizing the primal technology of heating food over an open fire.

Retrieved from https://www.amazon.co.uk/Catching-Fire-Cooking-Made-Human/dp/184668286X
In addition to the benefits mentioned, cooked foods were also safer to eat because the cooking process reduced the risk of food-borne pathogens and parasites, especially those commonly found on and in meats. Cooking gave early ancestral humans more energy per mass of food, reducing the amount of time spent on chewing, digesting, and hunting. Over time, smaller jaws and guts were naturally selected for as digestion became less critical, while cranial space and nutrients could be redirected to the ever-evolving brain [1]. Gradually, we humans became a species of cooks.
The First Artificial Ingredients
But, cooking does more than just breakdown and sterilize food. The process of cooking is a complex series of chemical reactions that transforms raw carbohydrates, proteins, and fats into new molecules rarely seen in nature. And some of those first manmade molecules include the amino acids that make up the proteins in meats and edible plant foods. I’ve considered the impact of these amino acids and made a few other inferences about the way cooked food could have tipped the evolutionary scales in our favor.
Amino acids come in two types, one labeled as “L” and one as “D”, which refers to the spatial orientation of the unique signature molecular groups attached to each one of the twenty amino acids. In organic and biochemistry, these two types are commonly referred to as mirror-image pairs. Their molecular formulas are identical, but each type is unique from its partner because one cannot be rotated in space and transformed into the other type. It’s a matter of how they’re oriented in three dimensions, much like how your left hand can’t be transformed into your right hand by rotation. They are mirror images of one another.

Mirror images of D and L-alanine. Bold lines represent groups out of the page, dash lines into the page. Retrieved from http://www.d-aminoacids.com/chirality/chirality.html
Now, the vast majority of the amino acids eaten by humans are in the L-form, and in fact, all of the proteins in our body are composed of L-form amino acids. However, when food proteins are cooked, some of those amino acids chemically transform into the D-form [2]. The process is known as racemization, and is driven by the high temperatures commonly found in cooking. And unlike the L-form, D-form amino acids cannot be metabolized by our cells, and therefore are biochemically inert, or worse, toxic. That’s because the enzymes and metabolic processes in our body were built to only handle the overly abundant L-amino acids found in nature. The result is a valuable chunk of the valuable amino acids found in foods are converted into a useless form. Because nature rarely produces D-amino acids, they are technically ‘unnatural’ and could be the first ‘artificial’ food ingredients produced by humans.
Role of Cysteine in the Human Body
Racemization happens to occur at different rates for different amino acids. Luckily, most amino acids do not go through racemization very quickly, otherwise we would end up very malnourished from cooked food since the L-amino acids will be turned into D-amino acids. But of all twenty amino acids, cysteine has the highest rate of conversion to its D-form when heated [2] and is commonly found in meat protein. Higher cooking temperatures, longer cooking times, changes in pH, and fermentation can all accelerate this process. Because of its high transformation rate, a large portion of the cysteine in cooked foods would be in the D-form. And while cysteine plays many critical functions in the human body, the D-form has little place in human metabolism.
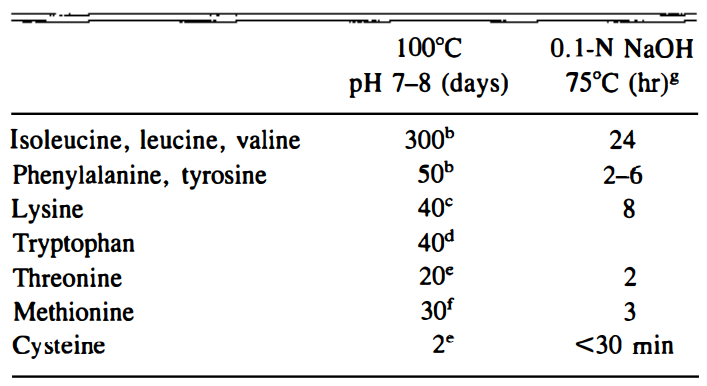
The racemization half-life rate of cysteine versus other amino acids for comparison [2].
Hydrogen Sulfide and the Human Brain
In the past several decades, a large body of research work has been focused on studying the physiological role of hydrogen sulfide [3-6]. Hydrogen sulfide is the gas responsible for the smell of rotten eggs. And while hydrogen sulfide is a toxic gas when inhaled at moderate concentrations in the air, researchers have discovered hydrogen sulfide serves as a gaseous signaling molecule between cells at pico- to nanomolar concentrations [4]. There is still some controversy over the exact amount that is physiologically relevant due to analytical limitations in instrumentational and molecular probe precision, but mounting evidence points to hydrogen sulfide playing roles as diverse as an antioxidant, a cytoprotectant, a signal molecule for wound healing, a synaptic transmitter, and a regulator of inflammation [6]. In low oxygen conditions, such as during anaerobic exercise, hydrogen sulfide can even momentarily replace oxygen in the mitochondria and continue producing energy through aerobic respiration. All cell tissues appear to have the enzymatic machinery to produce hydrogen sulfide from cysteine at various concentrations, but each tissue type responds differently to hydrogen sulfide influx. For example, neurons were found to strongly recover from oxidative stress and inflammation caused by oxygen radicals when administered physiological amounts of hydrogen sulfide [3].
Japanese researchers recently reported in a Nature Communications article a novel mammalian pathway for the production of hydrogen sulfide from D-cysteine [7]. While hydrogen sulfide can be generated from L-cysteine through three different enzymatic pathways available to all cells, the enzymes used to produce hydrogen sulfide from D-cysteine were only found in the kidneys and the brain. And when D-cysteine and D-cystine, the oxidized form of D-cysteine, were given to mice neuron cell cultures treated with toxic levels of hydrogen peroxide, they were able to more effectively save the neurons from cell death than L-cysteine, presumably because transport of D-cysteine into neurons is faster than L-cysteine. Moreover, they provided evidence that D-cysteine does not cause excitatory neuron damage to brain tissue, whereas L-cysteine administered directly to the brain has been shown to cause neuronal cell death. They further suggested that the only source of D-cysteine for humans is from the racemization process induced by heat- or alkaline-treated food, as mammalian cell tissues do not have the enzymatic equipment to racemize cysteine into the D-form [7].
A Biochemical Advantage
We can see here that cooking food gave a powerful biochemical advantage to early evolving hominids: it created a large reservoir of D-cysteine that could be directly shunted to and used almost exclusively by the brain. Our brain utilizes 20% of all the energy reserves of the body [8], which requires a massive turnover of ATP, the energy currency used by living organisms. As ATP is produced through the process of aerobic respiration in the mitochondria, a large amount of reactive oxygen species (ROS) is produced as a byproduct. These ROS can wreak havoc on neurons as they react and tear apart the fat-containing myelin sheath that acts as insulation to increase the rate of transmissions between neurons. The excess hydrogen sulfide generated from D-cysteine could have helped protect the ever-growing brain from damage from ROS. Much like coolant is needed to keep computer processors from overheating, D-cysteine protected the brain from over-oxidizing. In this instance, D-cysteine could even be considered the first brain nutritional supplement taken by every human across their lifespan, for eons. And perhaps the result of this unintentional million-year long experiment is the modern human brain.
Could it be that our evolution, if only in part, was spurred on by one of the world’s first artificial ingredients? If so, what would be real the distinction between a natural and artificial ingredient, this idea that there’s a strict dividing line between what nature created and what humans created?
Nowadays, the amount of nutrients that humans consume makes the amount of available cysteine irrelevant. Cysteine is found in most high protein foods, such as meat, eggs, milk, legumes, soybeans, oats, and wheat. Most people in the world can obtain enough cysteine to survive and thrive without suffering major setbacks in their neurological development because of the relatively high abundance of these foods thanks to modern agriculture. However, some mental and psychiatric disorders are hypothesized to be dependent on hydrogen sulfide concentrations; for example, individuals affected by Alzheimer’s have been shown to have severely decreased levels of hydrogen sulfide in their brain [9]. Further research will be needed to determine the true importance of D-cysteine in our evolution by considering the evolutionary genetics of the D-cysteine metabolizing enzymes, the relative importance of D-cysteine in human physiology, its role in brain development and health, and if there are specific nutritional requirements for this ‘unnatural’ amino acid, created using the oldest technology of humankind.

The process of oxidative phosphorylation, the last step in aerobic respiration. Retrieved from https://www.khanacademy.org/science/biology/cellular-respiration-and-fermentation/oxidative-phosphorylation/a/oxidative-phosphorylation-etc
Cooked Food on the Horizon
The possibility that cooking could have influenced the entire trajectory of our evolution is rather astounding. On the surface, cooking appears to be a mundane, everyday chore to feed our bodies. And even in modern times, some of us choose to cook as an artistic expression of our desire for delicious food, while others use cooking as a means of connecting with our friends and families. Cooking is no longer about survival for those living in the Western world. The modern technologies and food industries we have grown to depend on have allowed us to opt out of the need to cook for ourselves; what we eat is produced on a mass scale, and largely driven by the consumer demand of the general populace. However, if cooking really played a part in how we turned out as a species, the decisions made on the industrial, cultural, and personal level around the foods we choose to eat, and their means of production, may have just as much of an impact on our future evolution as any other transhumanistic technology, including artificial intelligence, cybernetics, nanotechnology, space travel, and genetic engineering [10].
And if Wrangham is right, we might have to end up changing the old maxim to: we are what we cook.
References
[1] Wrangham, Richard. Catching fire: How cooking made us human. Basic Books, 2009.
[2] Man, Eugene H., and Jeffrey L. Bada. “Dietary D-amino acids.” Annual review of nutrition 7.1 (1987): 209-225.
[3] Abe, Kazuho, and Hideo Kimura. “The possible role of hydrogen sulfide as an endogenous neuromodulator.” Journal of Neuroscience 16.3 (1996): 1066-1071.
[4] Li, Ling, Peter Rose, and Philip K. Moore. “Hydrogen sulfide and cell signaling.” Annual review of pharmacology and toxicology 51 (2011): 169-187.
[5] Wang, Rui. “Physiological implications of hydrogen sulfide: a whiff exploration that blossomed.” Physiological reviews 92.2 (2012): 791-896.
[6] Szabó, Csaba. “Hydrogen sulphide and its therapeutic potential.” Nature reviews Drug discovery 6.11 (2007): 917-935.
[7] Shibuya, Norihiro, et al. “A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells.” Nature communications 4 (2013): 1366.
[8] Swaminathan, Nikhil. “Why Does the Brain Need So Much Power?.” Scientific American 29.04 (2008): 2998.
[9] Eto, Ko, et al. “Brain hydrogen sulfide is severely decreased in Alzheimer’s disease.” Biochemical and biophysical research communications 293.5 (2002): 1485-1488.
[10] Owen, James. “FUTURE HUMANS: Four Ways We May, or May Not, Evolve.” National Geographic, National Geographic Society, 24 Nov. 2009, news.nationalgeographic.com/news/2009/11/091124-origin-of-species-150-darwin-human-evolution.html.

Bryan Quoc Le | 150 Food Science Questions Answered
IFTSA VP of Digital and Social Media (2019-2020)
Bryan is the author of 150 Food Science Questions Answered (Rockridge Press, 2020) and a Ph.D. candidate in Food Science at University of Wisconsin-Madison studying the health effects of garlic and onion flavors. He received his B.S. and M.S. in Chemistry at the University of California, Irvine. In another life, he walked 2,000 miles from California to Louisiana in six months, and learned that eating tuna and peanut butter every day was not meant for the average human body. After he met his wife, he learned that there was more to good food than canned goods and smoothies. While not juicing onions and pressing garlic, Bryan likes to run half-marathons, discover interesting cuisines with his wife, and help entrepreneurs develop great food products.






I love this! I’ve read before that cooking food allowed for a higher calorie consumption, and more energy = more brain power could develop. I never knew that the production of D-cysteine in cooked meat could also be another factor. Wow!
Absolutely brill. Brilliant!