By: Pat Polowsky
Sweetness. It’s one of the fundamental tastes that is crucial to just about every meal and eating occasion. We often think about dessert as being the focal point of sweet foods, but sugar often makes up the sensory backbone of many savory items as well. Barbeque sauce or ketchup without sugar would basically be salty vinegar. American white bread, much to Europe’s chagrin, would be missing a distinctive facet to its overall flavor profile. With that said, the usual suspects will always be top of mind. Soda, cookies, candies, oh my! All of these foods, and many others, rely heavily on sugars such as sucrose (table sugar), fructose (fruit sugar), lactose (milk sugar), etc.
The last couple of decades have brought a new wave of sweetness – alternative sweetness. To technically minded folks (and the FDA), these are termed “nonnutritive” sweeteners. These sweeteners, as the name implies, don’t contribute much to the nutritional needs of the body. Namely, caloric and glycemic responses, which makes them suitable for those who are avoiding caloric sweeteners for health reasons. These come in many different types and flavors (ha!), and can often encompass those found in nature (e.g. stevia or monk fruit extract) or those that are artificially generated (e.g. aspartame or sucralose). For brevity’s sake, we’re going to focus our discussion on the artificially derived ones and save natural nonnutritive sweeteners for another day. Also outside the scope of our discussion will be sugar alcohols (aka polyols), which also deserve a post all to their own.
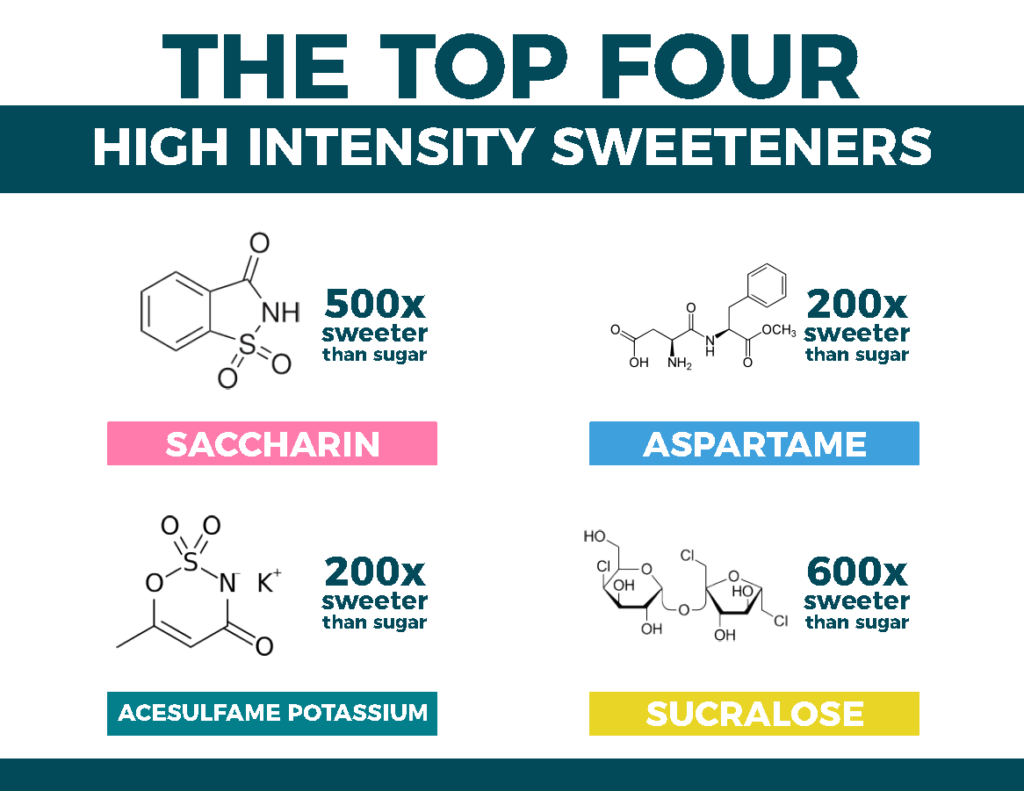
Before we dive in it’s worth mentioning that each one of these could also be described as “high intensity” sweeteners due to the fact they have a much higher perceived sweetness than ordinary table sugar/sucrose. We’re going to avoid the trappings of talking about how “good” or “bad” each nonnutritive sweetener is, and instead just objectively focus on learning some cool trivia for each one! Funny enough it’s not the actual sweetness perception that gives these away when used in products, but usually a bitter/metallic off-flavor or aftertaste. A partial remedy to this has been to use them in combination with each other. Their synergies help mask off-flavors and provide a more well-rounded sweetness profile. Let’s get started!
Saccharin
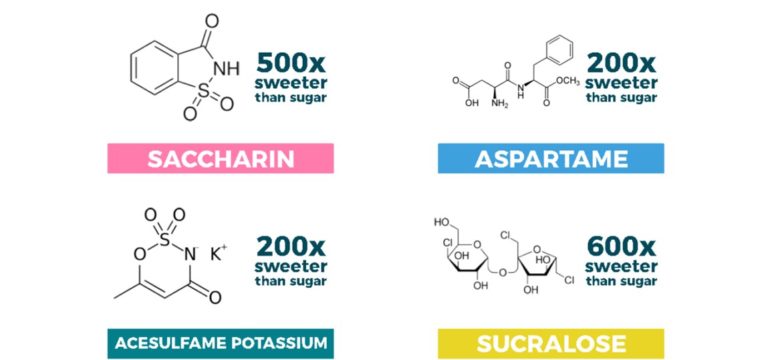
Scientifically speaking: Sodium saccharin (also known as benzoic sulfimide); approximately ~500 times sweeter than sucrose
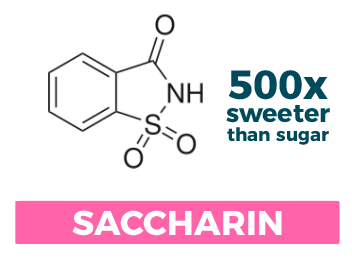
How you would recognize it: It comes in the pink packets
How it was discovered: One of the tastiest accidents in history! In the late 1800s a researcher at Johns Hopkins University, Constantine Fahlberg, was working on a very specific organic chemistry reaction relating to organic solvents. Unbeknownst to him, he had gotten some of a newly synthesized compound on his hand. It wasn’t until dinner, while eating his bread, that he realized just how sweet his discovery really was!
Uses and other tidbits: Saccharin is heat stable and has a reasonably long shelf-life. For this reason, it is sometimes used in conjunction with aspartame (see below) in diet soda formulations or other products where there is a worry that the main sweetener may lose effectiveness over time.
Aspartame
Scientifically speaking: Methyl ester of a dipeptide (two amino acids: aspartic acid and phenylalanine); approximately ~200 times sweeter than sucrose.
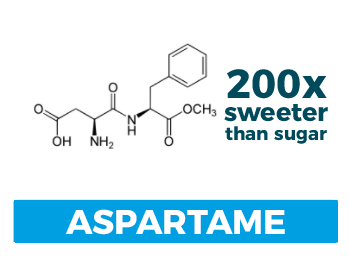
How you would recognize it: It comes in the blue packets
How it was discovered: In 1965 a pharmaceutical chemist, James Schlatter, was working on the chemistry of a human hormone: gastrin. Gastrin is a tetrapeptide (i.e. four amino acids) and aspartame was an intermediate step.
Uses and other tidbits: Aspartame was the darling of the diet soda industry for many years, but has recently fallen out of favor for more stable alternatives. Since it is protein-based, it is heat and chemically sensitive. This means it can’t survive extremely acidic conditions for prolonged periods and also can’t be used in cooking applications. What’s more, since aspartame contains phenylalanine, products that use it must carry a warning statement for folks who suffer from phenylketonuria (PKU).
Acesulfame Potassium
Scientifically speaking: Potassium salt of acesulfame, lovingly known as “Ace K”; approximately ~200 times sweeter than sucrose.

How you would recognize it: It’s not usually used on its own, but is very common in “zero”-type sodas.
How it was discovered: Another accidental discovery. A German chemist, Karl Claussat, was working on similar compounds when he had his eureka moment in 1967.
Uses and other tidbits: Ace K is very stable and is almost always used in conjunction with another nonnutritive sweetener. In fact, it’s often used with sucralose in “zero”-type colas since they play so well together on the palate! Fun fact: since it’s excreted at virtually 100% recovery from the kidneys, some researchers have used it to quantify how much urine is in swimming pools.
Sucralose
Scientifically speaking: It’s the most similar to sucrose thus far. It’s a sucrose molecule that has been partially chlorinated, although it’s approximately ~600 times sweeter than sucrose.
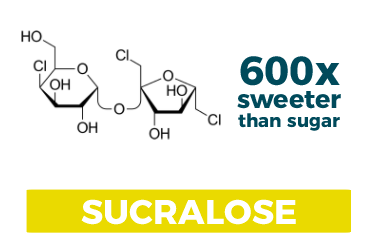
How you would recognize it: It comes in the yellow packets
How it was discovered: Another accidental discovery! Noticing a trend here? In 1976 a graduate student, Shashikant Phadnis, collaborating with Tate and Lyle misunderstood his boss’s instructions. Phadnis was supposed to “test” the newly formed compound, but instead he decided to “taste” it. And thus, one of the most prolific (and profitable) food products Tate and Lyle offers was born!
Uses and other tidbits: Sucralose, being so similar to sucrose, is very stable and can be used in many of the same applications. As such, it’s a common alternative to sugar in baked goods, candies, and other high-heat applications.
Conclusion
I think we’ve covered some good ground today. There are of course other high-intensity nonnutritive sweeteners on the market, but we’ve covered the major ones above. A key take away is that these serve a very specific purpose in many cases and the real “art” comes into play when mixing them together. On their own each one is quite unpleasant when tasted. But when combined together, or with the addition of a little bulking agent like cornstarch, or perhaps a pinch of salt, they can really shine!
Sources
Chemistry and Use of Artificial Intense Sweeteners
Sugar Substitutes: Artificial Sweeteners and Sugar Alcohols

Pat Polowsky | Linkedin | Website
Pat is a Ph.D. student in Agricultural Education & Communication at Purdue University. Most recently he was a senior product development manager on Walmart’s Private Brand team supporting the deli, entertaining, and gourmet categories. Previously he’s worked in Vermont and Wisconsin, focusing on cheese R&D, sensory science, and software development. In his free time, he runs cheesescience.org, an online educational toolbox for all things cheese. His passion in life is communicating food science in a visual and engaging way. Some folks like gardening on the weekend, whereas Pat enjoys making PowerPoint animations of casein micelles. He has his B.S. in Food Science from Purdue University and his M.S. in Food Science from the University of Vermont.





Leave a Reply