By Jacob Webster-Jones
Microwave ovens – or for short they are called microwaves – are one of my favorite kitchen appliances to use. When you need to heat up last night’s leftovers or that cup of coffee you didn’t drink quick enough a microwave is there to conveniently heat up your food as quick as possible without having to clean any extra pots or pans.
I think microwaves get a bad rap from most people because they don’t heat things exactly like an oven or stove. If you’ve ever tried to make cookies, breads, or really any baked goods in a microwave you’re typically rewarded with a really tough and sad imitation of their oven baked counterpart. While it’s true that microwaves don’t produce the same results, they’re actually amazing and offer their own benefits. Microwaves can quickly heat foods which can: (1) minimize the degradation of heat-sensitive nutrients such as vitamins A, B1, and B2; (2) offer more uniform heating to foods; and (3) provide more control over cook time which can be easily stopped and started by pressing a button thereby giving a higher level of control on heating than other thermal kitchen appliances. Additionally, on a commercial scale microwaves are used to precook and/or pasteurize foods.
History & Background
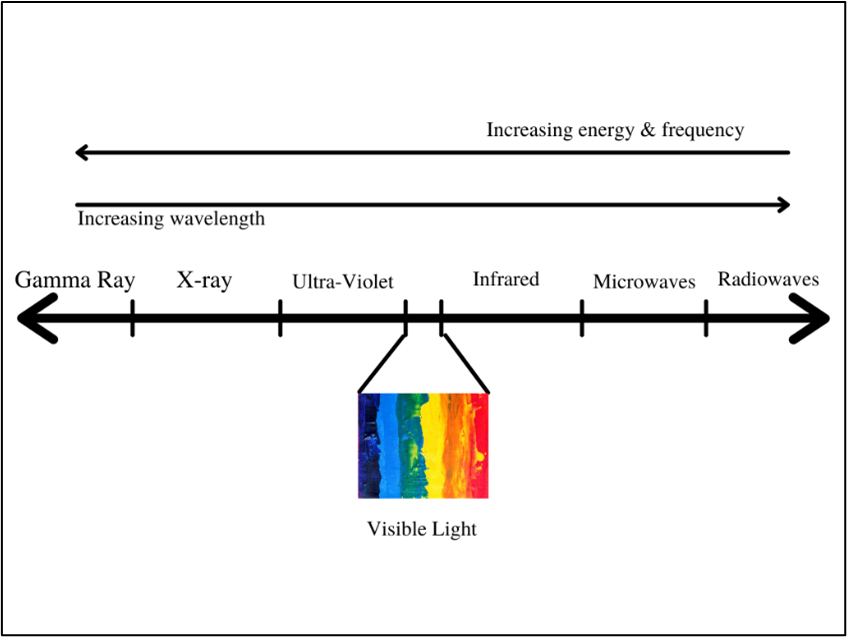
Photo by Jacob Webster-Jones
First, let’s take a little refresher on microwaves. A microwave refers to a particle of light (photon) with a frequency between 300 MHz and 300,000 MHz on the electromagnetic spectrum. As the frequency of a photon increases, the energy also increases while the wavelength decreases.
Like many other discoveries in food science, the use of microwaves to heat foods was an accident. In the 1940s, a scientist named Percy Spencer was contracted to work on a radar for the allied powers in WWII. While he was working close to one of the radars, he noticed that a peanut butter bar had slightly melted and proceeded to place corn next to the magnetron and made popcorn. Spencer manufactured some microwaves for commercial use in 1947 and received a patent in 1950. Between receiving the patent and its expiration in 1967 it wasn’t common to see microwaves in every household in the US; upon the expiration of the basic patent the market for home microwave ovens grew tremendously and soon most households had one in their kitchen.
Anatomy of a Microwave
A magnetron (generator), wave guides (aluminum tubes), distributor (rotating fan), and metal chamber make up a microwave. When a high voltage is applied to the magnetron it produces free electrons which in turn produce oscillating microwaves. These microwaves are then directed using the waveguides to either the chamber containing food or a stirrer (distributes the microwaves more evenly) then to the chamber.
Most home microwaves are 2450 MHz while industrial microwaves used for heating are at 915 and 896 MHz. The typical range of power varies from microwave to microwave but generally fall between 600 to 1200 watts. The higher the wattage of your microwave, the more power it can supply to the magnetron, and the less time it will take to heat up food.
Heating Mechanisms
If the microwaves that are produced don’t actually contain heat energy, then how does food get heated in a microwave? Foods contain molecules that have oppositely charged ends (polar molecule) which will orient themselves to their electric field. Earlier I mentioned that the microwave sitting in your kitchen at home most likely operates at 2450 MHz, this means that in one second the electric field is changing polarity 2450 times! The rapid movement caused by the polarity changes causes polar molecules, primarily water, to heat the food. This way of heating allows foods to heat more uniformly than traditional heating methods which involve the slow movement of heat from the outside to the inside of the food.
That being said there’s a good chance that while reheating some leftovers from the night before you’ve noticed that sometimes the middle of the food is still cool; while on paper the heating characteristics of microwaves are more uniform, this is only in certain conditions and is highly dependent on the food being microwaved.
The Cold Center

Image by EK Song from Pixaby
There are a number of factors that go into how microwaves are absorbed into a food that come from both the food itself and its container. Without getting into the weeds with symbols and equations; the basic properties that influence how any material will heat up in a microwave are its ability to absorb microwave energy, how efficient it turns the microwaves into heat, and the depth at which the microwaves penetrate. Both of these properties depend on factors including: frequency of the oven, moisture content, composition, density and temperature of the material.
This means that a food will heat differently in a microwave depending on what temperature it’s at. For example, water is much more absorptive and easily converts microwave energy into heat than ice. These factors also can be related to the depth to which microwave energy can penetrate a material. So, the reason your leftovers are still cold at after being in the microwave could be due to how the different ingredients absorb and convert microwave energy or the sheer volume of food is too thick for the microwaves to evenly heat the food.
Microwaves have quickly become staples in many home kitchens as a means of quickly and conveniently reheating or thawing cold foods. On an industrial scale these machines can be used to heat, dehydrate, or sterilize foods using less energy and time than traditional heating methods. As a result, this technology can be used to benefit food companies by reducing processing times, the environment with less overall energy usage, and consumers by providing safe and nutritious foods.
References
- Fellows, P. J. (2017). Dielectric, ohmic, and infrared heating. In Food Processing Technology Principles and Practice (4th ed., pp. 813–844). essay, Elsevier.
- Gordon, R. L. (2013). Packaging of Microwaveable Foods. In Food Packaging principles and practices (3rd ed., pp. 383–398). chapter, Taylor & Francis Group.
- P.J. Fellows, 20 – Dielectric, ohmic and infrared heating, Editor(s): P.J. Fellows, In Woodhead Publishing Series in Food Science, Technology and Nutrition, Food Processing Technology (Third Edition), Woodhead Publishing, 2009, Pages 581-609, ISBN 9781845692162, https://doi.org/10.1533/9781845696344.3.581.
- Tang, J., Mikhaylenko, G., & Simunovic, J. (n.d.). Microwave Pasteurization. http://microwavepasteurization.wsu.edu/MWH-delete/factsheet/index.html.
- Browne, Jack. “Microwave Energy Powers Many Industrial Applications.” Mircrowaves & RF, 10 Apr. 2017, www.mwrf.com/technologies/systems/article/21848252/microwave-energy-powers-many-industrial-applications.
 Jacob Webster-Jones | Linkedin
Jacob Webster-Jones | Linkedin
SMF Blog Writer
Jacob found his passion for food science while on an elementary school field trip to a R&D lab in San Antonio, Texas. Since then he has been interested in why food behaves the way it does and how it can be used to improve people’s quality of life. He is currently pursuing his bachelor’s degree in Food Science & Technology from Texas A&M University and is the current president of his school’s food science club and IFTSA chapter. After he graduates, Jacob plans on going to work for a few years before returning to start a graduate degree, his ultimate goal is to work in research and development for a flavor company. On the off chance he has free time you can find him experimenting in the kitchen or enjoying the outdoors.






Leave a Reply