
This hamburger patty was made from 20,000 muscle fibers grown from stem cells. Photograph: David Parry/EPA
Science fiction “meats” reality
Exploring the technology and challenges of cell-based meat
By – Wanxin “Maggie” Xue
Cell-based meat, as the name suggests, means meat that is grown from cultivating animal cells. The first cell-based beef patty was created by Maastricht University in 2013. The tasting session was on live television, marking the beginning of the cell-based meat era [1]. For the 10 years afterward, various companies have hopped on this concept. In 2020, the first cell-based lobster meat was launched by Shiok Meat, a Singapore company [2]. A couple of weeks ago, on Nov.16, 2022, cell-based meat is approved by the United States’ Food and Drug Administration (FDA), which is another milestone on this journey [3]. The science behind cell-based meat is more intuitive than it sounds. It can be broken down into four steps: cell line development, cell culture media formulation, scaffolding and bioprocessing. This is much like choosing the right seed and fertilizer for your garden and putting up the stick for tomatoes to climb on. (Fig. 1)
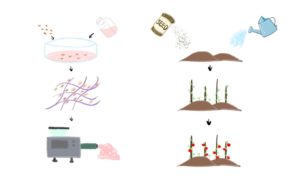
Fig. 1: Overview of process.
Cell line development
The goal of cell line development is not only to find the type of cell that’s most suitable for consumption but also to find a cell culture that dies easily after a few rounds of cell division. All natural cells have a limit to how many times they can divide, called the Hayflick limit. The Hayflick limit serves to prevent the increased likelihood of mutation occurrence after rounds of division. Much like the telephone game, the message becomes garbled after too many rounds. The downside of the Hayflick limit is obvious too, cells will die and that puts a limit to the age of all living beings [4]. Overcoming the Hayflick limit is important for cell-based meat production since we don’t want the cell to die easily. Scientists are trying to immortalize the cell, hoping to increase the proliferative capacity and achieve optimal yield. Stem cells, which can act as a parent cell for all other specialized cells, are especially interesting for this research. When we think about meat, it consists of a combination of muscle cells, fat cells, and fiber cells. Therefore, we want to utilize all these types of cells and finely combine them.
Cell culture media consideration
Cell culture plays the role of supporting cell growth and proliferation. Its composition can be separated into 2 parts: basal media and serum. Basal media provides enough nutrition to support cell viability, including basic nutrients like glucose, amino acids, salts, and vitamins. The serum includes substrates such as hormones and growth factors that promote cell proliferation. It is challenging to elucidate the most vital nutrients for cell growth, which has involved a lot of trial and error. Primarily, scientists use either a reductive or synthetic approach, which can also be understood as a top-down or bottom-up method, respectively. The reductive (top-down) method aims to find a solution that works well and deconstruct it one-by-one to determine the most necessary component, whereas the synthetic (bottom-up) method requires the knowledge to formulate a supportive media. The reductive approach plays a role in providing enough evidence to synthesize a viable recipe. Regardless of the method, the goal of the cell culture media is to develop a low-cost and scalable media formulation, given that 55-95% of the cost of cell-based meat production is from the media [5].
Scaffolding and bioprocessing
Hurdles to growing the cells, like physical pressure and the composition of the cell culture media, decide the fate of the cell. For example, if the stem cell receives more compression, it is more likely to turn into bone or cartilage. Conversely, it has a higher chance of turning into a tendon if it receives more stress from stretching [6]. The scaffold is a 3D porous structure that tries to mimic the extracellular environment for the cell to attach, proliferate and differentiate. The importance of scaffolding is like the stick for our garden tomatoes to grow on, given that the cells grow better when provided a support. A microcarrier is a scaffold that is commonly used for production. It looks like a tiny bead with a Cheetos PuffTM structure under the microscope. Usually, microcarriers are put in the bioreactor for the cell to attach. Depending on the design of the bioreactor, it can be a big tank with an impeller to stir and an air inlet to supply oxygen [7], or it can be a single-use disposable plastic bag sitting on a seesaw-like bench and mixed by the wiggling motion [8]. (Fig. 2)
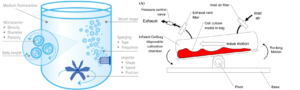
Fig. 2: Design of bio-reactors, stirred tank (l) and rocking platform (r).
When the cells are ready to harvest, they are washed off microcarriers with an enzyme called trypsin and collected [9]. Removing the cells from microcarriers sounds like a hassle, is there any easier solution? Scientists are looking into non-edible but degradable microcarriers, which can be degraded during the bioprocessing process. They are also working on making edible, food-grade and nutritious microcarriers, so that it can be a part of the final product. The target type of meat product is also a factor. If we want to create a highly structured meat product like steak or fillet, a highly vascularized scaffold is required. Scaffolds can be made from plant-derived, animal-derived, or even synthetic material. (Fig. 3) An interesting example is the decellularized plant, like spinach leaves and jackfruit. The fruit cells are washed off the structure, leaving the scaffold to support the desired fat and muscle cells. This method of scaffolding also finds utility in growing replacement body parts. A lab at the University of Ottawa grown a human ear on a scaffold carved to mimic the shape of an ear, seeded with human stem cells. This phenomenal tissue engineering achievement can potentially give us direction on cell-based meat technology [10].
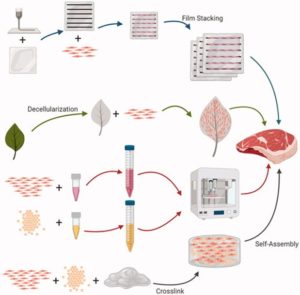
Fig. 3: Modes of scaffolding.
End-product considerations and future outlooks
After an animal is slaughtered, muscles are transformed into meat through a complex biochemical process called post-mortem. Changes in appearance, texture, flavor, and nutritional composition take place and result in a difference compared to cell-based meat. Therefore, the goal of cell-based meat is to be biologically equivalent to animal-based meat and mimic animal-based meat in terms of appearance, texture, flavor, and nutritional composition. For example, to mimic the appearance of blood, beetroot juice can be added. What’s more, “plant-based blood”, which is a fermented myoglobin called soy leghemoglobin can also be considered. Fat plays a major role in the flavor profile of different types of meats. However, it is hard to put adipose (fat) cells and muscle cells in the same matrix. In response to this hurdle, several startup companies specialize in cell-based fat production. The first cell-based meats have a dry texture because they lack overall connective tissue. Scientists are looking into designing better scaffolds to include connective tissue.
Nutrition is a big part of food choice consideration. Muscle cells, the major component of cell-based meat, don’t produce a range of nutrients like the ones found in animal meat. For example, conjugated linoleic acid, an important type of fatty acid, is only synthesized by ruminant gut cells. What’s more, the generated muscle and fat tissue are still in incomplete maturation resulting in a lower level of nutritional density. This is a major hurdle to overcome if we want to improve consumer acceptance of cell-based meats and provide a nutrient-rich product. The development of scaffolds that are edible, high in protein, and can support high cell density is the future direction of scaffold development [11].
Conclusion
It seems like a fairy tale to envision a world in which we can enjoy meat, but not harm the environment or kill animals. Cell-based meat technology could mitigate the environmental and ethical issues stemming from industrial animal farming and slaughtering. It makes us wonder what would be next. Would we be able to taste panda or mastodon meat soon? Just a thought…
References
[1] Jha, A. (2013, August 6). World’s first synthetic hamburger gets full marks for ‘mouth feel’. The Guardian. Retrieved November 20, 2022, from https://www.theguardian.com/science/2013/aug/05/world-first-synthetic-hamburger-mouth-feel
[2] Shiok meats launches the world’s first cell-based lobster meat in an exclusive tasting event. Shiok Meats. (2022, October 25). Retrieved November 20, 2022, from https://shiokmeats.com/news-features/shiok-meats-launches-the-worlds-first-cell-based-lobster-meat-in-an-exclusive-tasting-event/
[3] FDA. (2022, November 16). FDA spurs innovation for human food from Animal Cell Culture Technology. U.S. Food and Drug Administration. Retrieved November 20, 2022, from https://www.fda.gov/news-events/press-announcements/fda-spurs-innovation-human-food-animal-cell-culture-technology
[4] Bartlett, Z. (2014, November 14). The Embryo Project Encyclopedia. The Hayflick Limit | The Embryo Project Encyclopedia. Retrieved November 20, 2022, from https://embryo.asu.edu/pages/hayflick-limit
[5] Yao, T., & Asayama, Y. (2017). Animal-cell culture media: History, characteristics, and current issues. Reproductive Medicine and Biology, 16(2), 99–117. https://doi.org/10.1002/RMB2.12024
[6] Janaszak, M. M., Wolfe, R. P., & Ahsan, T. (2016). Biomechanics in Stem Cell Manufacturing. In Stem Cell Manufacturing (pp. 27–42). Elsevier Inc. https://doi.org/10.1016/B978-0-444-63265-4.00002-9
[7] Schnitzler, A. C., Verma, A., Kehoe, D. E., Jing, D., Murrell, J. R., Der, K. A., Aysola, M., Rapiejko, P. J., Punreddy, S., & Rook, M. S. (2016). Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: Current technologies and challenges. Biochemical Engineering Journal, 108, 3–13. https://doi.org/10.1016/J.BEJ.2015.08.014
[8] Valdiani, A., Hansen, O. K., Johannsen, V. K., & Nielsen, U. B. (2020). An efficient bioreactor platform for scaling up the proliferation of Nordmann fir’s (Abies nordmanniana) somatic embryos. International Journal of Environmental Science and Technology, 17(3), 1425–1438. https://doi.org/10.1007/S13762-019-02556-4/FIGURES/6
[9] Karnieli, O. (2016). Bioreactors and Downstream Processing for Stem Cell Manufacturing. In Stem Cell Manufacturing (pp. 141–160). Elsevier Inc. https://doi.org/10.1016/B978-0-444-63265-4.00006-6
[10] Seah, J. S. H., Singh, S., Tan, L. P., & Choudhury, D. (2021). Scaffolds for the manufacture of cultured meat. Https://Doi.Org/10.1080/07388551.2021.1931803.
[11] Ng, S., & Kurisawa, M. (2021). Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomaterialia, 124, 108–129. https://doi.org/10.1016/J.ACTBIO.2021.01.017
About the Author:
Wanxin Xue | Linkedin
 Wanxin is currently pursuing her undergraduate degree in Food Science at the University of Guelph. Wanxin also goes by the name Maggie, because her parents met each other at Maggi soy sauce plant and fell in love, which set the tone for Wanxin picking Food Science as her study and career choice. Wanxin is also the President of the Food Science club at the University of Guelph, enjoying building the community of foodies and helping people know more about Food Science. Growing up in China and coming to Canada to study, Wanxin develops a strong appreciation towards different cultures. She loves traveling, making new friends and exploring local culture through the cuisine. In her free time, you can also find her juggling.
Wanxin is currently pursuing her undergraduate degree in Food Science at the University of Guelph. Wanxin also goes by the name Maggie, because her parents met each other at Maggi soy sauce plant and fell in love, which set the tone for Wanxin picking Food Science as her study and career choice. Wanxin is also the President of the Food Science club at the University of Guelph, enjoying building the community of foodies and helping people know more about Food Science. Growing up in China and coming to Canada to study, Wanxin develops a strong appreciation towards different cultures. She loves traveling, making new friends and exploring local culture through the cuisine. In her free time, you can also find her juggling.






Leave a Reply