BY: MORGAN REASE
Here in California and throughout the US it is once again time for farmers’ markets. Walking through the market, I like to survey the booths from a distance looking for produce that catches my eye. Deep red tomatoes, dark greens, vibrant berries, squashes — I want them all! There’s an adage that you eat with your eyes first, and it’s truer than you may realize. Vision plays a very big part in how you perceive the quality and flavor of a food. But what causes these different colors and what is color anyway?
Different pigments in food
The wide array of colors in fruits and vegetables (we won’t be covering the colors of meats or fish in this article) is the result of a few classes of chemicals: chlorophyll, flavonoids, anthocyanins, betalins, and carotenoids.
Table 1: Common pigments and their properties. Useful for applying science at home!
| Pigment | Colors | Foods | Solubility | Effect of pH |
| Anthocyanins | Red-Purple-Blue | Berries, Red Cabbage, Flowers | Water soluble | Very dependent on pH |
| Betalins | Red – Yellow | Beets, Amaranth | Water soluble | No color shift, most stable at pH 4-5 |
| Carotenoids | Red – Yellow | Carrots, Squash, Tomatoes, Peppers | Fat soluble | |
| Flavonoids | White – Yellow | White Cauliflower | Water soluble | |
| Chlorophyll | Green | Leafy Greens, Herbs, Green Cabbage | Water soluble | Less stable under acidic conditions |
| Diarylheptanoids | Yellow | Turmeric | Fat/alcohol soluble. Poorly soluble in water | In water: Stable, but less soluble, under acidic conditions |
Chlorophyll
Chlorophyll is the chemical compound that makes plants look green. It also plays a significant role in the plant’s ability to photosynthesize! Spinach, kale, celery, lettuce, and herbs are all green from chlorophyll. If you could see a molecule of chlorophyll, you’d see a cyclic structure with a long tail; in the center of it all is a magnesium ion.[4] That little ion is very important, and you’ll see why further down the page.

The basic structure of a flavonoid is C6C3C6, but there is a huge array of different compounds within this classification. (image source: https://en.wikipedia.org/wiki/Flavonoid)
Flavonoids
Flavonoids are a actually a whole class of compounds rather than just one, like chlorophyll was. They often contribute white or yellow colors to foods, though when they are oxidized they can contribute darker colors like browns and blacks.[4]

The basic structure of a flavonoid is C6C3C6, but there is a huge array of different compounds within this classification. (image source: https://en.wikipedia.org/wiki/Flavonoid)
Anthocyanins
Anthocyanins are classified within flavonoids, but we’re going to consider them separately due to their substantial and unique impact on the color of so many foods. Most red or purple foods are red or purple because of anthocyanins. Berries? Those have anthocyanins. Red Cabbage? Anthocyanins again. Anthocyanins can exist in four pH dependent structures, two of which are actually colorless.[4] The equilibrium of the 4 structures varies by which specific anthocyanin you look at. In general, high pH = blue color, low pH = red color, but the colorless compounds can also make up a large percentage. It’s important to understand how the anthocyanins in a specific food change with pH if you want to utilize the color for something, a liquor infusion for example (we’ll talk about that later too).

A basic structure for an anthocyanin. See how it shares the C6C3C6 structure with flavonoids? (image source: https://en.wikipedia.org/wiki/Anthocyanin)
Red cabbage has a very stable set of anthocyanins; its juice can even be used as a pH indicator.

After boiling cabbage to extract the anthocyanins, pH of the resulting liquid can be adjusted to achieve a colorful spectrum.
Image Source: my own photo
Betalins
Betalins are far less common than anthocyanins, though they can have similar colors. In fact, foods that contain one do not contain the other (they are mutually exclusive). Compared to anthocyanins, betalins are in relatively few foods, among which are beets and amaranth. The two types of betanins are betacyanins (which are red) and betaxanthins (which are yellow). Hence, red and yellow beets. Under the right conditions, betacyanins can actually turn into betaxanthins![4] It’s not likely to happen in your food in any meaningful quantity, even if you tried. The food would just look less red. Betalins are most stable in the pH range of 4-5, but color of the compound is not really affected by changing pH as it is with anthocyanins.

Betanin, a type of betacyanin, is responsible for the red color of beets. (image source: https://en.wikipedia.org/wiki/Betalain)
Carotenoids
Carotenoids are extremely common around the world. Carotenoids give carrots their color, as well as squashes, oranges, tomatoes, and much more. When leaves turn all those pretty colors in the fall, those are carotenoids! Many leafy greens are also full of carotenoids, but we can’t see them because they also contain a lot of green chlorophyll.[4]

Beta-carotene, a precursor compound to vitamin A, is a carotenoid. (image source: https://en.wikipedia.org/wiki/Beta-Carotene)
Diarylheptanoids
Diarylheptanoids are not as widespread as other pigment compounds listed above. However, I want to include them here because of one very important, very colorful food. Turmeric! Turmeric gets its color from a compound known as curcumin.[3, 5] Turmeric powder is used to give curry powder and yellow mustard their intense yellow color. Like carotenoids, curcumin is fat soluble. However, if you want to dissolve it in water, it is less soluble under acidic conditions than alkaline, but is less stable in an alkaline environment.[5]

Image source: http://cancerres.aacrjournals.org/content/canres/59/3/597/F1.large.jpg
Foods can contain more than one kind of pigment. Cauliflower, for example, has varieties that can appear white (flavonoids), green (chlorophyll), orange (carotenoids), or purple (anthocyanins).

Multi-colored cauliflower (image source: https://greenblender.com/smoothies/author/elizahurwitz/page/2)
What is color anyway?
Vision is made possible by light being reflected at our eyes from the objects around us. We can only see light of certain wavelengths. This range of wavelengths is referred to as the visible spectrum. The stuff we can’t see includes X-rays, microwaves, radio waves, and UV light.[2] All of it is the same thing: Energy! But it’s beyond the ability of our eyes to process it all. Probably a good thing too as I can already see plenty to distract me. Colors are just specific wavelengths of light. Purples are around 400 nanometers long, while reds are closer to 700 nanometers long. When light strikes an object, that light is either absorbed or reflected. If an object appears blue, that means the object is absorbing all light except for the blue light, which is being reflected back into your eyes.[2] White objects reflect all wavelengths, while black absorbs all wavelengths.
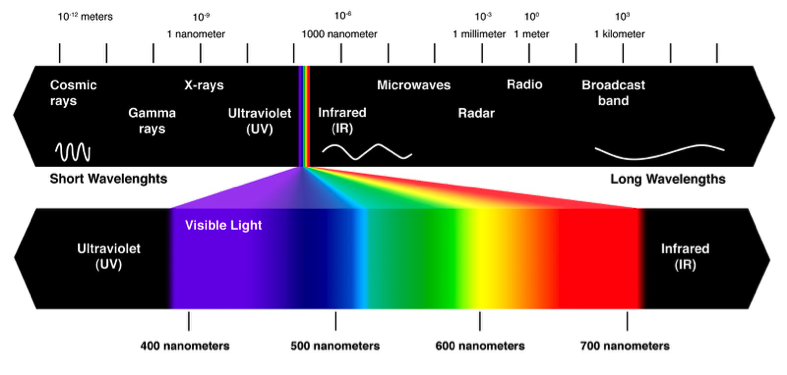
A visualization of the visible spectrum of light and non-visible forms of energy (image source: http://www.theskepticsguide.org/humans-seeing-infrared-light-kind-of)
A brief lesson in quantum mechanics
As strange as it may seem, chemicals can only absorb energy in specific amounts.[1] What amount of energy can be absorbed is a function of – among other things – the structure of the chemical compound.[1,6] Hence, different compounds appear as different colors to us! You can actually calculate what color a chemical will be by using quantum mechanics. Below is an equation and all the numbers you need to determine the absorbance of beta-carotene, from which you can infer its color.[1,4] Don’t be scared! It’s easier than you might think.
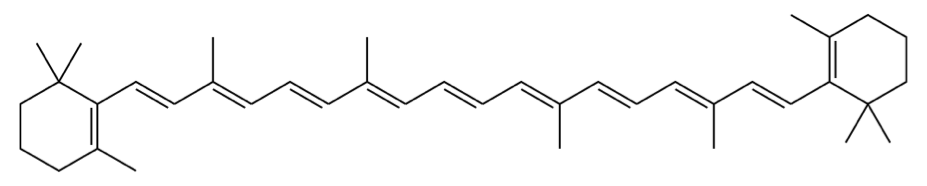
beta carotene molecule (image source: https://en.wikipedia.org/wiki/Beta-Carotene)
λ = wavelength
λmax = wavelength of maximum light absorbance
λmax = (8*me*[L^2]*c)/h(N+1)
me (mass of an electron) = 9.11 x 10-31 kg
L (length of the conjugated system in meters) = 1.8 x 10-9 m
c (speed of light) = 3 x 10^8 m/s
N (number of double bonds in conjugated system) = 22
h (planck’s constant) = 6.626 x 10-34 m^2kg/s
If you did math it out, you should see a λmax in the blue/purple region of the visible spectrum. You should recall from earlier that because that light is absorbed, beta carotene is reflecting the other portions of the spectrum (red-orange) at you!
*if you’re lame and don’t want to bust out your calculator, the answer to quantum mechanics problem is 464nm (it’s an approximation, not exact)*
Kitchen applications
All this information is pretty cool. But why not put our newly acquired knowledge to use? Dan Barber, the chef at Blue Hill Stone Barns uses carotenoid rich peppers as feed for chickens to give them a deeply colored yolk. The egg yolk is where most of the fat is in an egg, peppers get their color from carotenoids, which are fat soluble. Delicious, brilliantly colorful eggs. Science!
When people cook leafy greens, they often turn from bright green to a muddy brown color. This is from the loss of magnesium (see? Told you this little guy was important) from the chlorophyll to form a different compound, called pheophytin.[4] You can preserve the green color at home by blanching your greens in boiling, mildly alkaline water. The high heat will deactivate the enzyme that catalyzes the conversion of chlorophyll to pheophytin, while the alkaline water will raise the pH. Conversion to pheophytin is more rapid under acidic conditions.[4] If you have calcium hydroxide on hand (labeled simply as Cal in mexican markets), not only would that raise the pH of your water, but the calcium ions can also help preserve the green color and prevent the veg from getting too soft.

image source:(https://www.instagram.com/p/BUQFTNuFhMO/?taken-by=chefdanbarber&hl=en)
In the craft cocktail and beverage scene, a lot of different plants are used for infusions. Knowing how best to extract a color, or alter that color, would be very useful. A personal project of mine for several months has been using science to make color changing cocktails. Yeah, you read that right. Color. Changing. Cocktails.
There are a lot of different fruits and vegetables you could use for this, so don’t feel that you have to follow this recipe exactly. You have the knowledge to experiment! Below is simply what has worked best for me so far.
Anthocya-Gin & Tonic
- Gin (or any other colorless liquor) 100g
- Butterfly Pea Flowers (available on Amazon) 5g
- Tonic Water
- Simple Syrup
- Lime
Combine the gin with the flowers and let them infuse for 3 hours. Strain the flowers out and pour the gin back into the bottle. Add 2 oz gin to a glass (make sure it’s clear so you can see the pretty colors), add ice, and simple syrup. The color should still be bright blue unless you’ve added something acidic. Now, when you’re ready, add the tonic water. The tonic is acidic (yes, even though it tastes bitter) and will turn the drink into a lovely pink! Squeeze in a lime. Beautiful and delicious. Wow your friends and impress a date with your science knowledge and cool drinks.
So cheers! Make yourself a drink, cut into some fruit, and appreciate the amazing molecular world around you the next time you walk through the market.
Works Cited
- Ames, James. “Quantum Mechanical Partical in a Box.” CHE 107B Lecture. UC Davis, Davis. 2015. Lecture.
- Cassidy, Rachel, and Regina Frey. “”I Have Seen the Light!” Vision and Light-Induced Molecular Changes.” Vision and Light-Induced Molecular Changes. Washington University, Nov. 2000. Web. 31 May 2017.
- “Curcumin.” Linus Pauling Institute. N.p., 03 Jan. 2017. Web. 08 June 2017.
- Damodaran, Srinivasan, Kirk Parkin, and Owen Fennema. Fennema’s Food Chemistry. 4th ed. BOCA RATON: CRC, 2008. Print.
- Stankovic, ChemiIvancal. “CURCUMIN: Chemical and Technical Assessment (CTA).” Joint FAO/WHO Expert Committee on Food Additives 61 (2004): n. pag. FAO. Food and Agriculture Organization of the United Nations. Web. 8 June 2017.
- “University of Massachusetts Chemistry Exam Key.” Chem/Biochem 471 Half Exam 6 12/10/10 (2010): n. pag. University of Massachusetts. Web.






Why am I getting 516 for the calculation? Please someone help me.
Thank you
Looks like the formula got a little mushed when the article went up, so it’s a bit hard to read now. Sorry about that! I think you’re probably multiplying the length of the box (L) by 2 instead of squaring it. If you fix that you should get 464.
We fixed the formula so it should read correctly now. Thanks for catching the mistake. 🙂