By Abbie Sommer
In my house growing up we always had a well-stocked pantry of staple ingredients. Of course, there were the standard baking ingredients needed to make my mom’s famous chocolate chip cookies. There was dried pasta, beans, canned tomatoes, and an assortment of spices. I also remember a small plastic container of these mysterious golden cubes of flavor, chicken bouillon cubes.
But what is in these little cubes that can turn water into broth? In this edition of What’s in my food, I will be going into detail about the most common ingredients in bouillon and why they are there.
Sugar and Salt
The four most classically recognized tastes that we perceive are sweetness, saltiness, sourness, and bitterness. Of those, saltiness is the most common taste associated with chicken broth.
The first ingredient in many bouillon products is salt. Salt of course adds saltiness and is one of the main constituents of the broth flavor. Salt can also help with the stability of the product. High levels of salt will keep any microbial growth at bay.
Additionally, you will find sugar as one of the top ingredients. Sugar helps round out the flavor and may also help to provide some microbial stability. Corn syrup solids may be added to the mix as well. The main component of these is glucose, as opposed to the sucrose in table sugar. Glucose is less sweet than sucrose and may contribute to the body and mouthfeel of the broth when reconstituted without adding too much additional sweetness.
Water and Fat
In most bouillons chicken fat is added, but fat can also come in the form of palm oil or corn oil. With all of these dry ingredients, something has to hold it all together. You may then ask, why not use water and keep it lower in fat?
There are two types of solids in which food ingredients can exist, crystalline and amorphous. Crystalline solids have an ordered structure (think table sugar or salt). These crystals do not absorb water, but water present on the surface can start to dissolve the crystals, a phenomenon known as deliquescence; or water can lead to bridging between the crystals. When the water starts to evaporate the salt that was dissolved leads to hard bridges between the crystals. In later stages this can lead to agglomeration (crystals starting to stick together) and compaction (really sticking together and not easily coming apart).
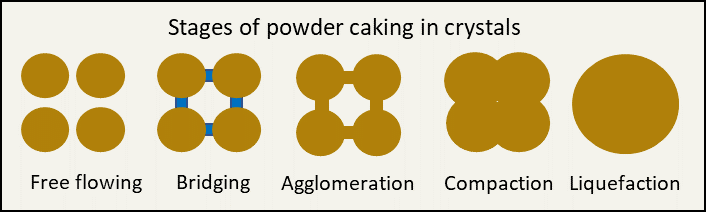
Graphic by Abbie Sommer
Amorphous ingredients do not have that ordered structure (think cotton candy). In amorphous ingredients the water can cause sintering, a similar sticking together of particles1. This is kind of like when the cotton candy starts to turn into a sticky mess on a humid day.
For bouillon, while a compact cube is the goal, it is also important that the cube can dissolve. When the particles stick together too tightly, it makes it harder for the cube to mix into water to make broth.
Fat, however, does not lead to caking but rather acts as a binding agent. Fat also acts as a carrier to some of the aromatic flavor compounds and can contribute to the silky mouthfeel of the finished broth.
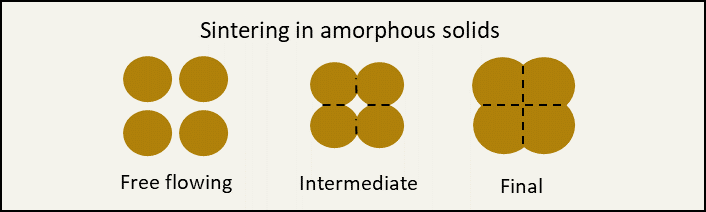
Graphic by Abbie Sommer
Color
Characteristic of bouillon, especially the chicken cubes, is a bright yellow color. This color is achieved by a mix of natural or artificial colorants that are added to the mix. Turmeric was commonly seen in the ingredient list, as was caramel coloring. These are both natural colorants. In some cases, the natural color annatto was added. Some brands will also include artificial colors like Yellow 5 and Yellow 6.
Stability
To keep these bouillon cubes stable and safe, a few other ingredients are sometimes added. When mixing crystalline (salt and sugar) and amorphous (spices, maltodextrin, etc.) ingredients, caking behavior can be increased even at low moisture contents2. Anti-caking agents such as silicon dioxide, maltodextrin, and starches help to keep this from occurring. Additionally, stabilizing agents like TBHQ, sulfites and citric acid help to prevent against rancidity of the fat and oxidation of other flavor compounds. This keeps these cubes shelf stable. See the table below for a deeper dive into how these stabilizers work and their safety in foods.
| Ingredient | Structure | How it works | Safety |
| Tertiary Butyl-
hydro- quinone (TBHQ) |
 |
TBHQ is an excellent antioxidant in fat. Exposure to light, oxygen, or heat can lead to fat oxidation and the formation of radicals. These then lead to rancidity and off flavors. TBHQ helps scavenge those radicals so they cannot promote rancidity. | At the levels present in foods, TBHQ has been classified as safe after testing by the Joint FAO/WHO Expert Committee on Food Additives3. |
| Sulfites |  |
Sulfites can be used to reduce browning of the vegetables in bouillon and/or inhibit microbial growth4. | Sulfites can lead to adverse reactions in some people, especially those with asthma. Sulfites do have GRAS (generally recognized as safe) status by the FDA and can be consumed by most without problems4. |
| Citric Acid |  |
Citric acid is of course an acid. This is one way that it works, brining the pH down low enough that microbes cannot grow. It also acts as antioxidant and can help prevent browning5. | Citric acid is naturally in fruits and vegetables. Citric acid was given GRAS status due to its historical use and safety. A recent article did cite four case reports of manufactured citric acid causing adverse reactions, possibly due to allergic reactions to the mold Aspergillus used to produce it. More research needs to occur on this front, as the article included few subjects (4) and was observational, not experimental6. |
The Umami Ingredients
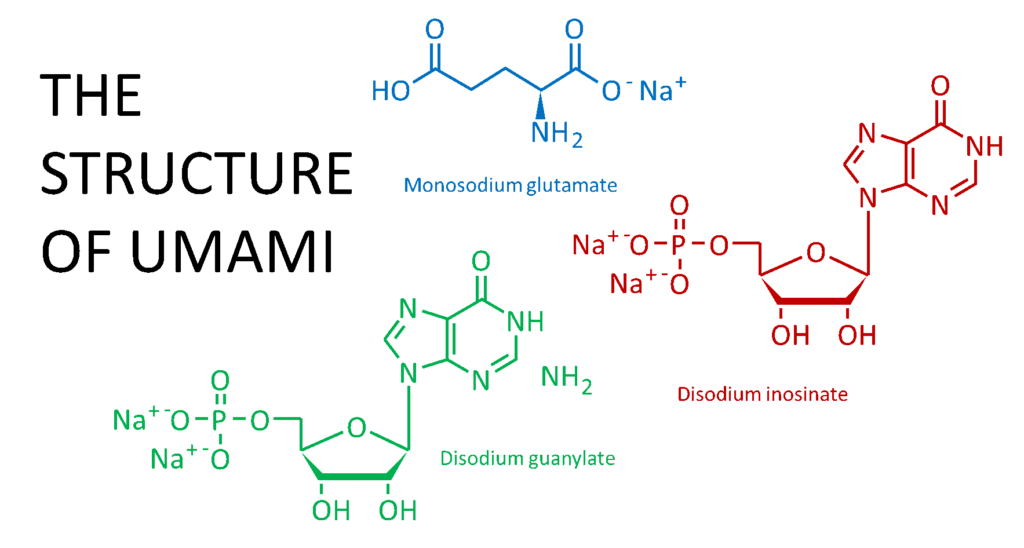
Graphic by Abbie Sommer
One of the main characteristics of chicken broth, stock, and bouillon cubes is the savory, meaty flavor. Monosodium glutamate (MSG), disodium inosinate, and disodium guanylate are often added to bouillon cubes to enhance this umami flavor. Amino acids, such as glutamate, and nucleotides, including inosinate and guanylate, are known to provide umami flavor7.
Amino acids are the building blocks of proteins in plants and animals. As for the nucleotides, you may remember guanine as being one of the four building blocks of DNA. Inosine is a product of breaking down adenosine, another nucleotide. All of these are naturally found in plants and animals.
It has also been shown that including both the MSG and disodium inosinate or guanylate creates an even more intense umami flavor7. Bryan Quac Le writes more in depth about the discovery and significance of this substances in his Science Meets Food Article “In Search of Flavor”.
Yeast extract or hydrolyzed yeast extract are also used as a source of these umami compounds such as nucleic acids, amino acids, and peptides5. Additionally, many of the bouillons contained hydrolyzed soy or corn proteins. These hydrolyzed vegetable proteins have been broken down into their component amino acids and can also be a source of umami5.
In meat-flavored bouillons, you will often find some meat as well. In the chicken flavor this is often in the form of dried, roasted, or extracted chicken. The concentration of meat is generally higher in a bouillon paste rather than the dried cubes. Beyond providing meaty flavor, the meat itself is a source of umami as protein is the source of these peptides and amino acids. This includes glutamic acid, which is the main amino acid in many proteins7.
Flavoring ingredients such as garlic, onion, and other vegetables may also contribute to the umami.
Talking about MSG will inevitably lead to the question, is it safe? The Joint FAO/WHO Expert Committee on Food Additives (JECFA) conducted a study on the safety of MSG and related compounds such as glutamate. It was found that MSG and related compounds do not represent a hazard to health at the levels present in food8. They also disproved the role of MSG in so called “Chinese restaurant syndrome”, with symptoms said to include flushing, tightness of chest, or difficulty breathing8. The FDA also conducted its own study on the safety of MSG and found that there were no proof of adverse effects of large doses of MSG in the general population8.
Seeing a list of ingredients that have long and confusing names can be intimidating but knowing what they do and where they come from can help demystify things. I think kids’ pantries start to look like their parents as they get older. Mine has all of the baking ingredients that were common in my mom’s and you will also find a small plastic container of bouillon cubes ready to be made into a quick broth on demand.
References
(1) Aguilera, J. M.; del Valle, J. M.; Karel, M. Caking Phenomena in Amorphous Food Powders. Trends Food Sci. Technol. 1995, 6 (5), 149–155. https://doi.org/10.1016/S0924-2244(00)89023-8.
(2) Voelker, A. L.; Sommer, A. A.; Mauer, L. J. Moisture Sorption Behaviors , Water Activity-Temperature Relationships , and Physical Stability Traits of Spices , Herbs , and Seasoning Blends Containing Crystalline and Amorphous Ingredients. Food Res. Int. 2020, 136 (July). https://doi.org/10.1016/j.foodres.2020.109608.
(3) van Esch, G. J. Toxicology of Tert-Butylhydroquinone (TBHQ). Food Chem. Toxicol. 1986, 24 (10–11), 1063–1065.
(4) Taylor, S. L. Sulfites in Foods: Uses, Analytical Methods, Residues, Fate, Exposure Assessment, Metabolism, Toxicity, and Hypersensitivity. In Advances in Food Research; Chinchester, C. O., Mrak, E. M., Schweigert, B. S., Eds.; Academic Press, Inc.: London, 1986.
(5) Igoe, R. S. Dictionary of Food Ingredients, Fifth.; Springer: New York, 2011; Vol. 7. https://doi.org/10.1007/978-1-4419-9713-5.
(6) Sweis, I. E.; Cressey, B. C. Potential Role of the Common Food Additive Manufactured Citric Acid in Eliciting Signi Fi Cant in Fl Ammatory Reactions Contributing to Serious Disease States : A Series of Four Case Reports. Toxicol. Reports 2018, 5, 808–812. https://doi.org/10.1016/j.toxrep.2018.08.002.
(7) Yamaguchi, S.; Ninomiya, K. Umami and Food Palatability. In Flavor Chemistry: 30 Years of Progress,; Teranishi, R., Wick, E. L., Hornstein, I., Eds.; Kluwer Academic: New York, 1999; pp 423–431. https://doi.org/10.1093/jn/130.4.921s.
(8) Walker, R.; Lupien, J. R. The Safety Evaluation of Monosodium Glutamate. J. Nutr. 2000, 130 (4), 1049S-1052S. https://doi.org/10.1093/jn/130.4.1049s.
*Featured image by Karolina Grabowska from Pexels
Abbie Sommer | Linkedin | Website
SMF Blog Writer
After graduating with a B.S. in Food Science from Purdue University, Abbie decided to move one state over to pursue a Masters from Ohio State. Her research is focused on soy-based functional foods for use in clinical trials. When she’s not making thousands of soft pretzels (for science, of course), you can find her training for half marathons or experimenting in the kitchen. Recently, Abbie has developed a passion for sourdough and treats her starter like a child. She also has a recipe blog (Sommer Eats) as well as an Instagram account (sommer_eats), where she posts somewhat healthy but always delicious recipes.






Leave a Reply